Clinical Trial led by EMBARC Reveals Potential of Novel Antibody Therapy against Pseudomonas in Patients with Bronchiectasis - Results of the GREAT-2 Study
- Proof of concept study achieves primary endpoint of a reduced bacterial load with the 500mg dose
- Treatment with gremubamab achieved statistical significance on multiple secondary endpoints compared with placebo
- Participants reported statistically significant and clinically meaningful improvements in quality of life across multiple domains with both doses of gremubamab
- Both doses of gremubamab were well tolerated
- Results suggest that antibacterial antibody therapy could offer a promising approach for targeting Pseudomonas aeruginosa, with a potential to improve patient outcomes and reduce antibiotic dependency in bronchiectasis
Results from the GREAT-2 trial have been announced today at the American Thoracic Society (ATS) annual conference in San Francisco, providing evidence for the efficacy and safety of gremubamab, an investigational bi-specific antibody developed by AstraZeneca to potentially prevent exacerbations in people with Pseudomonas aeruginosa infections. The multi-centre, randomised, double-blind, placebo-controlled study met its primary endpoint; gremubamab significantly reduced bacterial load compared to placebo in patients with bronchiectasis and chronic Pseudomonas aeruginosa infections. Gremubamab was well-tolerated in the study, with rates of adverse events and serious adverse events being similar in the gremubamab and placebo groups.
Eligible participants were adults >18 years of age with a confirmed clinical diagnosis of bronchiectasis and P. aeruginosa airway infection confirmed by positive sputum culture or PCR test. Eligible participants were randomised in a 1:1:1 ratio to receive either 1500mg or 500mg gremubamab, or placebo. Intravenous infusions were administered every four weeks over a 12-week period, followed by a 12-week follow-up phase.
37 participants were enrolled in this proof-of-concept multi-centre study. The primary endpoint was change in colony-forming units (CFU) as a measure of bacterial burden. Secondary outcomes included time to first exacerbation and patient-reported quality of life and symptom measures. Quality of life was assessed using three distinct, clinically-validated questionnaires: Quality of Life Bronchiectasis (QoL-B), Bronchiectasis Impact Measure (BIM), and St. George’s Respiratory Questionnaire (SGRQ). As a proof of concept study, a 1-sided p-value <0.1 was considered statistically significant.
The topline efficacy and safety results are below.
The 500mg dose successfully met the trial’s primary endpoint of significant reduction in bacterial load after 12 weeks of treatment compared to placebo (-1.25 log-CFU [80% CI: -2.33 to -0.16]; 1-sided p=0.071; ANCOVA). A directional trend was found with the 1500mg dose but failed to reach statistical significance (-0.66 log-CFU [80% CI: -1.71 to 0.39]; p=0.2).
Statistically significant and clinically meaningful reduction in SGRQ total score from baseline to EoT (Day 84) were observed in both the 500mg group (-12.12[80% CI: -17.72 to –6.52]; p =0.008) and the 1500mg group (-10.83[80% CI: -16.72 to –4.93]; p=0.02). Additional significant improvement was also seen across multiple domains of the BIM, SGRQ and QOL-B questionnaires at both doses. Notably, the 1500mg dose group also demonstrated a significantly prolonged time to first exacerbation compared to placebo.
The study was supported by the European Respiratory Society (ERS) clinical research collaboration programme and funded by AstraZeneca. This was an investigator-initiated collaborative study run by the EMBARC network with support from the European Lung Foundation (ELF) patient advisory group who provided patient input.
“It is very encouraging to see such a large improvement in quality of life, and the significant effect on exacerbations in this proof of concept study,” said Professor James Chalmers (study Chief Investigator; MBChB, Ph.D, Professor and Consultant Respiratory Physician at the School of Medicine, University of Dundee, UK).
“One treatment group showed a 12-point improvement on the St. George’s Respiratory Questionnaire, which is far greater than the 4 point change that we would consider clinically relevant for patients – an effect we consider potentially life-changing. If this can be replicated in larger studies, this could be a significant breakthrough in developing treatments for bronchiectasis, particularly in managing Pseudomonas infection.”
Bronchiectasis is a chronic respiratory condition characterised by permanent airway dilation, impaired mucociliary clearance, and recurrent infection. Pseudomonas aeruginosa, an opportunistic and often multidrug-resistant bacteria, represents a major clinical challenge in this population.
Previous EMBARC studies have demonstrated its high prevalence in sputum cultures from patients with bronchiectasis and have established its association with increased airway inflammation, exacerbation frequency, hospitalisation, reduced quality of life, and higher mortality risk.
There are currently no approved therapies specifically indicated for either bronchiectasis or P. aeruginosa infection.
“It is exciting to see the scientific promise of an antibody approach against this bacterial pathogen,” said Tonya Villafana, Vice President, Franchise and Medical & Scientific Affairs, Vaccines & Immune Therapies, AstraZeneca. “AstraZeneca is progressing the clinical development of several monoclonal antibodies targeting bacterial pathogens, including AZD0292, a half-life extended version of gremubamab targeting P. aeruginosa in patients with bronchiectasis.”
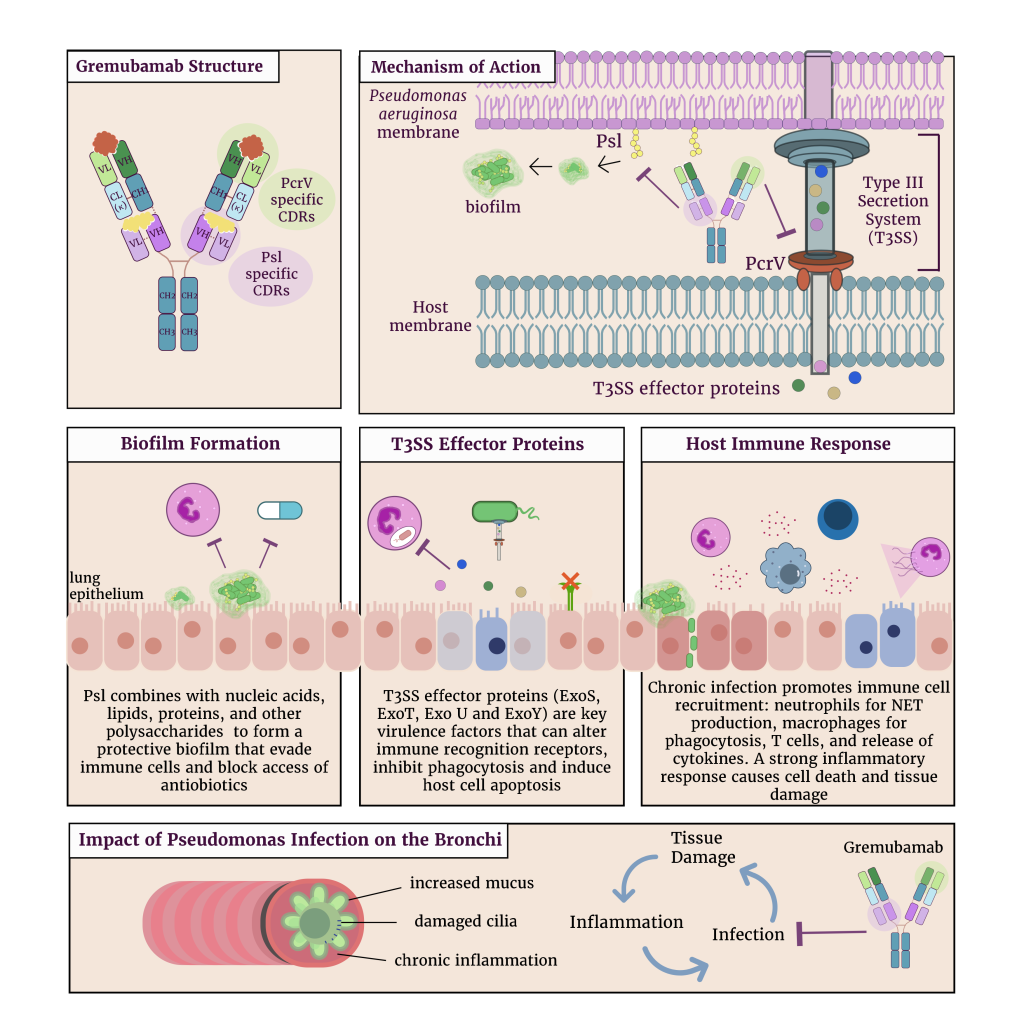
Gremubamab is a novel bispecific IgG1κ monoclonal antibody designed to simultaneously target two key virulence determinants of P. aeruginosa: the PcrV protein, a critical component of the type III secretion system (T3SS), and Psl, an exopolysaccharide involved in biofilm formation and immune evasion (Figure 1). By inhibiting both T3SS-mediated toxin delivery and biofilm-associated protection mechanisms, gremubamab aims to neutralise bacterial virulence and enhance host immune function.
According to ERS guidelines, the current standard of care for P. aeruginosa-colonised patients is the use of nebulised antibiotics. Treatment with nebulised antibiotics can relieve symptoms, but the use of broad spectrum antibiotics may have a negative impact on the lung microbiome by reducing overall microbial diversity and promoting the emergence of antibiotic-resistant bacteria.
“This research represents an important step toward a novel approach to infection management, demonstrating the potential of targeted, non-antibiotic therapies” said Dr Merete Long, Lead Scientist of the study from the University of Dundee, School of Medicine.
“Such strategies are particularly critical in the current era of rising antimicrobial resistance, where conventional antibiotics are increasingly limited in their effectiveness. The results of this study are encouraging, and show the value of collaborative, translational research to find new potential treatment approaches”